The State of Continuous Glucose Monitoring (CGM) in 2025: Breakthroughs, Devices, and What Patients Should Know
Introduction
Continuous glucose monitoring (CGM) has transformed diabetes care over the past decade, moving from intermittent finger-sticks to near-continuous, real-time glucose data.
In 2024–2025 several technological leaps—longer sensor life, implantable devices, deeper pump integration, and progress in non-invasive sensing—are reshaping how clinicians and people with diabetes manage glycaemia. This article explains the most important innovations, clinical implications, and practical advice to choose and use CGM systems effectively.
What is a CGM and why it matters
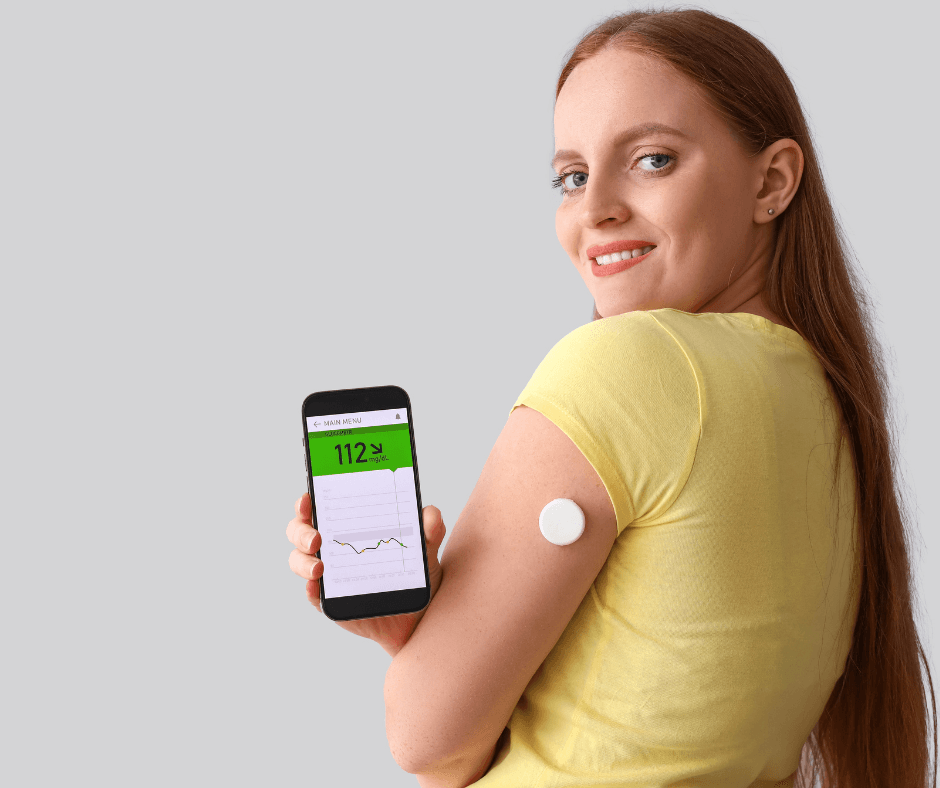
A Continuous Glucose Monitor (CGM) measures glucose in interstitial fluid at regular intervals (often every 1–5 minutes) and transmits readings to a smartphone or receiver. Key benefits include: real-time trends, alerts for hypo- and hyperglycaemia, and the ability to inform insulin dosing and lifestyle decisions. Common search queries related to CGM include: “best CGM 2025”, “CGM implantable”, “Dexcom vs FreeStyle Libre”, and “closed-loop insulin pump”.
Market leaders and form factors in 2025
Major CGM products on the market include patch-style sensors (e.g., Abbott FreeStyle Libre series), wearable stick-on sensors (e.g., Dexcom G7), and implantable systems (e.g., Eversense). Recent product updates have focused on accuracy, user comfort, app integration, wear time extension, and simplified calibration workflows. Natural Endocrinology Specialists+1
Key breakthroughs and trends (2024–2025)
- Year-long implantable CGMs — Implantable sensors that remain under the skin for months have arrived. The Eversense 365 (one-year implantable CGM) received regulatory clearance and represents a major step toward long-term monitoring for adults with diabetes. This reduces sensor replacement frequency and improves adherence for people who struggle with skin irritation from adhesive patches. diaTribe+1
- Longer wear time for wearables — Leading patch and wearable CGMs have extended approved wear windows (for example regulatory approvals and label updates have supported 15-day wear versions in 2025). Longer wear increases convenience and reduces recurring device costs. Investors
- Tighter pump + CGM integration: closed-loop systems — Automated insulin delivery (AID) systems—or “artificial pancreas” solutions—are increasingly mature. Commercial systems (iLet, Omnipod, Tandem Control-IQ and others) now integrate with modern CGMs to automate insulin delivery and reduce burden for users. These systems are being expanded to broader populations, including many patients with type 2 diabetes. diabetotech.com+1
- AI and predictive analytics — Cloud-based analytics and on-device machine learning provide better glucose forecasts, smarter alerts, and individualized coaching. Expect more personalized time-in-range optimization and earlier detection of glycaemic patterns that require clinical attention.
Clinical and regulatory updates to watch
- Expanded indications and pregnancy — Professional guidelines and standards of care in recent updates have broadened CGM recommendations, including more use during pregnancy and in people with type 2 diabetes receiving insulin. Clinicians are increasingly encouraged to consider Continuous Glucose Monitoring for glycaemic management and risk reduction. Diabetes Journals
- Insurance & access — Coverage policies vary by country and payer. New devices (longer-wear CGMs or implantables) can change coverage dynamics; clinicians should document medical necessity and quality-of-life improvements to support reimbursement.
Practical advice for patients and clinicians
- Choose the device that matches patient priorities: wear time, accuracy, skin tolerance, smartphone integration, and cost.
- For people using insulin: pair CGM with an AID system if accessible—closed-loop therapy often improves time-in-range and reduces hypoglycaemia. diabetotech.com
- Be cautious about non-FDA/CE-approved “glucose” claims from wearable watches; rely on validated CGMs for medical decisions. Verywell Health
- Regularly review time-in-range, glucose variability, and pattern reports with a diabetes team.
Implications for the next 3 years
Expect incremental improvements in accuracy, miniaturization, data interoperability (apps + EHRs), and cost reduction. Implantables and longer-wear sensors will broaden options for users who prefer low-touch solutions .
While AI and decision-support will increasingly assist clinicians in personalizing therapy.
Q&A — Clear, short answers for readers (use at end of article for SEO / user help)
Q: What is the most accurate CGM in 2025?
A: Accuracy varies by model and use case; market leaders all report excellent accuracy in clinical evaluations—selection depends on wear preference and integration needs. Natural Endocrinology Specialists+1
Q: Is there a CGM that lasts a year?
A: Yes . the Eversense 365 is an implantable CGM . Approved for one-year sensor life and its reducing the need for frequent sensor replacement. diaTribe+1
Q: Can my smartwatch measure glucose non-invasively?
A: Not reliably for medical decisions. Some research prototypes are promising, but consumers should avoid relying on unapproved smartwatch claims and use validated CGMs for treatment decisions. Nature+1
Q: What is an artificial pancreas?
A: An artificial pancreas combines a CGM, an insulin pump, and control algorithms to automatically adjust insulin delivery by reducing hypo and hyperglycaemia and improving time-in-range. Commercial systems are increasingly available and integrated with major CGMs. diabetotech.com

Thank you for this article it was very helpful.keep writing ❤️❤️❤️
MERCI POUR VOTRE COMMENTAIRE